The diet is crucial in the management of kidney diseases. A well-planned and balanced diet can help slow the progression of kidney diseases, manage symptoms, and improve overall health.
The Kidney
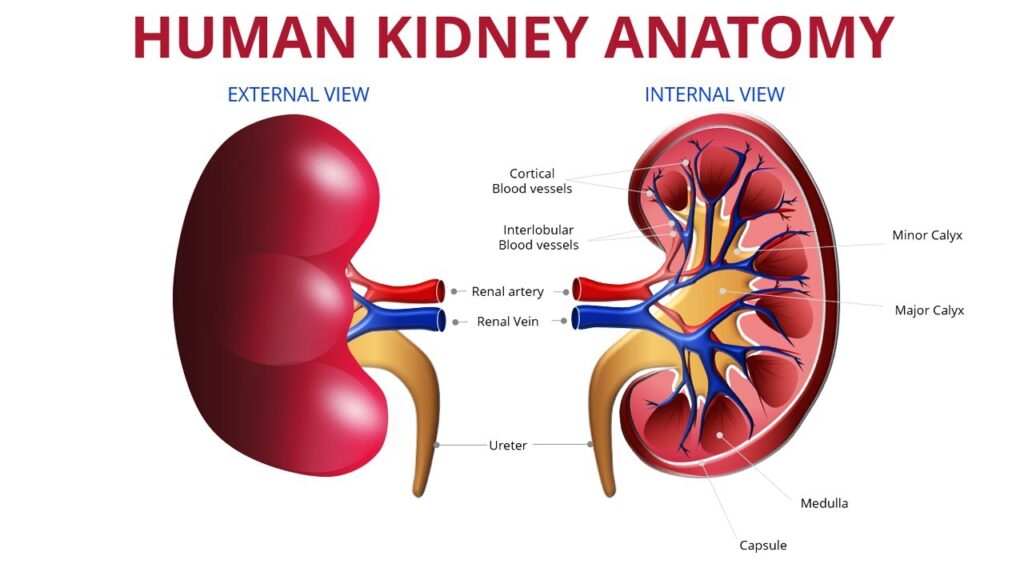
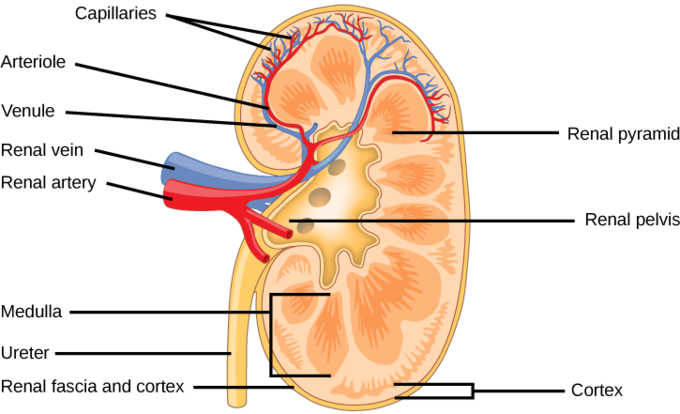
The kidney are two bean shaped organs that filter the extra water and wastes out of blood. They lie against the back of the abdominal wall, outside the peritoneal cavity, just above the waistline in the lumbar area. They are highly surrounded by a huge network of blood vessels and are segmented into three main regions:
- Renal cortex – outer region which contains about 1.25 million renal tubules),
- Renal medulla – a collecting chamber in the middle region which
- Renal pelvis – a cuplike cavity in the inner region which receives urine through the major calyces.
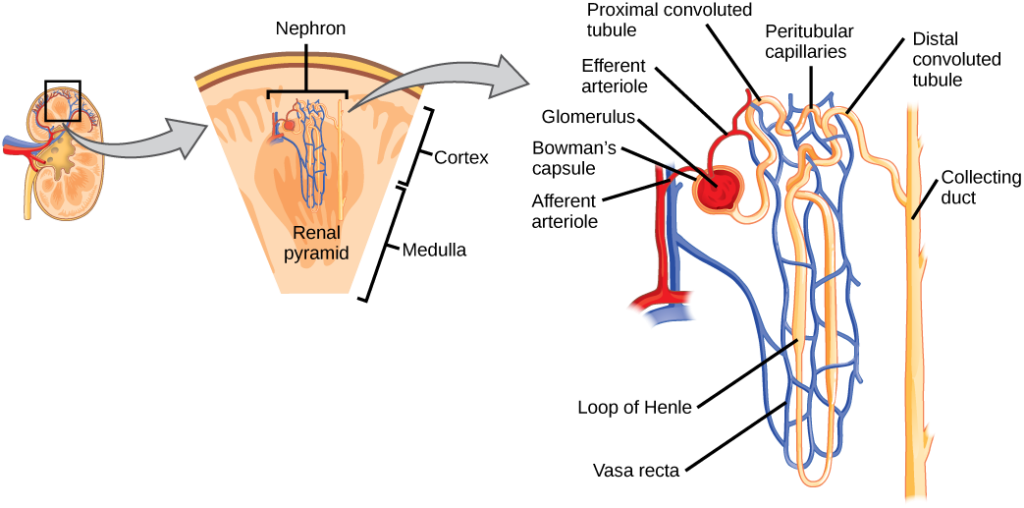
The Nephron are the filtration units in the kidneys. These essentially carryout the kidney functions:
Functions of the Kidney
- Waste excretion: The kidneys filter out toxins, excess salts, and urea, a nitrogen-based waste created by cell metabolism.
- Water level balancing: As water intake decreases, the kidneys adjust accordingly and leave water in the body instead of helping excrete it.
- Blood pressure regulation: The kidneys need constant pressure to filter the blood. When it drops too low, the kidneys increase the pressure. One way is by producing a blood vessel-constricting protein (angiotensin) that also signals the body to retain sodium and water.
- Red blood cell regulation: When the kidneys don’t get enough oxygen, they send out a distress call in the form of erythropoietin, a hormone that stimulates the bone marrow to produce more oxygen-carrying red blood cells.
- Acid regulation: If the body is to function properly, it needs to keep a healthy balance of various salts.
Kidney Diseases
There are various complications that are associated with kidneys. The following are the common kidney diseases:
- Kidney Stones (Nephrolithiasis)
- Chronic kidney disease
- Acute Renal Failure
- Nephrotic syndrome
Kidney Stones (Nephrolithiasis)
Kidney stone, or nephrolithiasis, is the presence of renal calculi caused by a disruption in the balance between solubility and precipitation of salts in the urinary tract and in the kidneys. Kidney stones develop when urine becomes “supersaturated” with insoluble compounds containing calcium, oxalate, and phosphate resulting from dehydration or a genetic predisposition to over-excrete these ions in the urine. The incidence is at peak among males aged 20 and 30 years (Han, Segal, Seifter, & Dwyer, 2015).
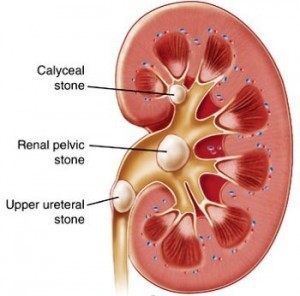
Kidney stone formation
When concentration is above the normal solubility a crystal starts to form; nucleation begins. The levels of urinary super-saturation of the different solutes determine the specific types of stones. Nevertheless, according to Han et al., (2015), stone formation may result due to the following conditions that favour development of kidney stones:
- Increased urinary crystalloids can form nucleuson the existing surface and supersaturate urine.
- Decreased inhibitors – Magnesium complexes with urinary oxalate while citrate complexes with calcium making them unavailable for stone formation. When they are in low supply, one is at a higher risk of stone formation.
- Increased promoters – such as uric acid when in high concentrations in the urine will promote the nuclei to start stone formation.
- Dehydration – If the patient is dehydrated, he or she will have low urine output and therefore the urine can be supersaturated.
- Urine pH – Urine pH is very important for the formation of some types of stones. A low urine pH has more insoluble uric acids concentration; therefore, the risk of uric acid stone is higher. High urine pH can increase the risk of CaP stones.
- Diet – supplies most of these elements that enhance or reduce the chance of stone formation
- Medication – some medications may cause supersaturation of urine with these elements when they contain the elements in excess. E.g. Furosemide: decrease urinary volume while Na bicarbonate: increase urinary Ca.
Types of kidney stones
According to Han et al., (2015), there are 5 types of stones that commonly affect people. These include:
- Calcium oxalate/ mix
- Calcium phosphate (brushite)
- Uric acid
- Struvite (Mg ammonium phosphate)
- Cystine
Approximately 70-80% of kidney stones are composed of calcium oxalate and calcium phosphate. Of the rest, 10% are struvite, 10% of uric acid; and less than 1% are composed of cystine or are diagnosed as drug-related stones. Calcium and uric acid stones are more common in men; women have more struvite stones (Han et al., 2015).
Food safety knowledge is for all!

Every consumer deserves to have high quality and safe food. …Read more!

Clinical Diagnosis of Kidney Stones
Non-obstructing kidney stones produce no symptoms or signs apart from hematuria. However, the kidney stone may cause severe pain, usually accompanied by nausea, vomiting and hematuria (renal colic) when it passes into the ureter. Patients may also complain of urinary frequency and urgency. Diagnosis may include:
- Laboratory tests may show a leukocytosis which may be due to a stress response or infection. The urinalysis will have red blood cells, white blood cells and occasionally crystals.
- Imaging studies are critical in making the diagnosis. Initial evaluation includes obtaining a non-contrast helical CT, which can accurately visualize the size and location of the stones.
For medical and nutrition evaluation of kidney stones, the dietitian should evaluate dietary intakes of calcium, oxalates, sodium, protein (both animal and plant), dietary supplements and fluid intake. Several dietary factors can increase risk of the stone formation, including sodium, protein, potassium, calcium, magnesium. These constituents can be modified depending on the types of different stone risks (Han et al., 2015).
Dietary recommendations to prevent kidney stones
The dietitian’s role in nephrolithiasis care is very important. The dietitian should assess nutritional risk factors by dietary intake assessments and provide therapeutic recommendations based on dietary risks. Dietary assessment is very important both in treating and preventing stone formation.
According to the National Kidney Foundation, (Han et al., 2015), the following dietary recommendations are ideal:
Table 1. Dietary recommendation to prevent kidney stones
| Nutrients | Recommendation |
| Ca Oxalate Na Protein Fluid Vitamin D Vitamin C | 800–1,200 mg/d 40-50 mg/d 2,000–3,000 mg/d 0.8–1.4 g/kg/d >2.5 L/d Low dose if vitamin D insufficiency or deficiency (1,000 IU/d) Dietary Reference Intake |
Medical Treatment & Prevention
Dietary modification – Important to reduce the recurrence rate of urinary stones. Of greatest importance in reducing stone recurrence is an increased fluid intake. Absolute volumes are not established, but increasing fluid intake to ensure a voided volume of 1.5-2.0 L/day is recommended.
Surgical Treatment – In the acute setting, forced intravenous fluids will not push stones down the ureter. Urgent surgical intervention is indicated in a patient with an obstructed, infected urinary tract, worsening renal function, intractable pain or vomiting or obstruction of a solitary or transplanted kidney.
Acute Renal Failure
Acute kidney injury (AKI) is the sudden decrease in kidney function that compromises the normal regulation of fluid, electrolyte, and acid-base homeostasis (National Kidney Foundation Primer on Kidney Diseases (Sixth Edition), 2014). It is characterized by a rapid (hours to weeks) decline in the glomerular filtration rate (GFR) and retention of nitrogenous waste products such as blood urea nitrogen and creatinine (Meersch, Volmering, & Zarbock, 2017).
It occurs commonly among intensive care unit (ICU) patients, and about 5% of ICU patients require renal replacement therapy (RRT) (Heung & Yessayan, 2017). The term AKI recognizes that smaller decrements in kidney function that do not result in overt organ failure, are of substantial clinical relevance and are associated with increased morbidity and mortality (Meersch et al., 2017).
AKI is an increasingly common condition that is associated with long-term health outcomes. Recent epidemiologic and observational studies underscore the association of an episode of AKI with long-term adverse outcomes such as chronic kidney disease, end-stage renal disease, cardiovascular events, and premature death.
In addition, other recent studies have demonstrated that AKI, particularly when severe or persistent, is associated with all-cause mortality, CKD, ESRD, cardiovascular events, and reduced quality of life (Silver & Siew, 2017). Nevertheless, it is considered as an acute condition, potentially reversible with full restitution if patient survives the acute phase of the disease.
Food safety knowledge is for all!

Every consumer deserves to have high quality and safe food. …Read more!

Causes of acute kidney injury
AKI can occur in a variety of settings. Risk factors include comorbidities such as higher age, CKD, diabetes, chronic obstructive pulmonary disease, heart failure/cardiac decompensation, as well as acute medical conditions like sepsis, major surgery, mechanical ventilation and hemodynamic instability (Meersch et al., 2017). Acute kidney failure can occur for many reasons. Among the most common reasons are:
- Acute tubular necrosis (ATN)
- Severe or sudden dehydration
- Toxic kidney injury from poisons or certain medications
- Autoimmune kidney diseases, such as acute nephritic syndrome and interstitial nephritis urinary tract obstruction
Consequences of acute kidney injury
AKI causes the following:
- Fluid and electrolyte imbalance
- Oliguria – low urine outputs
- Hypokalemia and hyperphosphatemia
- Uremia
Management of acute kidney injury
Timely recognition of patients at risk or with possible AKI is essential for early intervention to minimize further damage and improve outcome. Initial management of patients with suspected and persistent AKI, should include thorough clinical assessment of all AKI patients to identify reversible factors including the fluid volume status, potential nephrotoxins and an assessment of the underlying health of the kidney.
Based on these assessments early interventions to provide appropriate and adequate fluid resuscitation while avoiding fluid overload, removal of nephrotoxins and adjustment of drug doses according to level of kidney function derangement are important. The judicious use of diuretics for fluid overload / cardiac decompensated patients and introduction of early enteral nutritional support need to be considered to improve outcomes in AKI (Shetty, Yang, Bagga, & Chakravarthi, 2017).
Potential opportunities to improve care include:
- Closer monitoring of kidney function, management of CKD complications, blood pressure control, (Silver & Siew, 2017).
- Gene based therapies for kidney regeneration (Janssen et al., 2016)
Gene based therapies range from restoring gene function in genetic kidney diseases to steering complex molecular pathways in chronic kidney disorders, and can provide a treatment or cure for diseases that otherwise may not be targeted (Janssen, Arcolino, Kok, & Mastrobattista, 2016).
According to Chen & Busse, (2017), management of AKI may involve or include the following:
- Fluid Management – The mainstay of management of AKI from a macro- vascular perspective revolves around judicious use of i.v. fluids. To maintain adequate renal macrovascular perfusion, liberal administration of i.v. fluid has historically been standard of care.
- Renal Flow Modifiers – Alteration in microvascular renal blood flow at the level of the single nephron has been implicated in AKI. Disease states such as ischemiareperfusion injury, hypercalcemia, and hepatorenal syndrome, as well as iatrogenic factors, including the use of certain medications (NSAIDs, cyclooxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers) can result in an inadequate trans- glomerular pressure gradient and a reduction in glomerular filtration. Novel therapeutics such as angiotensin II and adenosine analogues seek to address these microvascular issues.
- Angiotensin – Angiotensin II is an octapeptide with multiple functions. In the kidney, angiotensin II participates in the regulation of the release of aldosterone, the maintenance of sodium and water homeostasis, and the release of vasopressin. It has been found to cause constriction of efferent arterioles to a greater degree than afferent arterioles, thereby increasing intra- glomerular flow and augmenting the transglomerular pressure gradient.
- Adenosine Antagonists – Adenosine has both intra and extra-renal effects that differ depending on the type of receptor it manipulates vascular tone to regulate adequate oxygen delivery to local tissues. In the kidney, adenosine can cause a reduction in GFR in response to hypoxia or an increased level of sodium chloride in the tubule via the constriction afferent arterioles.
- Antioxidants -There is an increasing amount of data describing the role of free radical oxygen species in the development of AKI. There are some potential therapies that directly target free radicals in the prevention of AKI caused by these clinical scenarios. E.G. Alpha-Lipoic Acid, Selenium, Sodium-2-Mercaptoethane Sulphonate, Propofol and Curcumin.
- Inflammatory Mediators – Kidney injury is associated with the release of inflammatory cytokines and chemokines that in turn attract immune cells such as neutrophils, macrophages, and natural killer cells. As such, the various proteins, such as Alkaline Phosphatase, Dipeptidylpeptidase-4 Inhibitors and Sphingosine 1 Phosphate Analogues, involved in these complex pathways could serve as targets for innovative therapies.
Treatment of acute kidney injury
Peritoneal dialysis (PD) – a suitable method for renal replacement therapy patients (Ponce et al., 2015).
Nephrotic syndrome
In nephrotic syndrome, the glomerular filtration barrier fails to retain protein leading to proteinuria, hypoalbuminaemia, hyperlipidemia and oedema (Liebeskind, 2014; Zolotas & Krishnan, 2016). The majority of the patients are younger than 6 years of age (80%) and there is a male predisposition (2:1). In childhood, most cases are idiopathic and treatment with steroids leads to complete remission of symptoms (Zolotas & Krishnan, 2016).
Causes of nephrotic syndrome
Minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), and membranous nephropathy (MN) are the most common causes of the nephrotic syndrome (Chopra & Thomas, 2014). This systemic disorder may also result from diverse underlying causes, including renal diseases such as IgA nephropathy, and Oracongenital predisposition.
Alternatively, nephrotic syndrome may develop due to diabetes, amyloidosis, viral infections, malaria, pre-eclampsia, systemic lupus erythematosus, or other disorders that affect the kidneys. Immune complex injury of the glomerulus by cancer antigens may cause membranous nephropathy. It has also been associated with nonsteroidal anti-inflammatory drugs, gold, lithium, mercury, interferon-b-1a, pamidronate, penicillamine, or heroin use.
Diagnosis of nephrotic syndrome
Diagnose nephrotic syndrome by demonstrating edema, proteinuria >3.5 g/24 hours, hypoalbuminemia, and hyperlipidemia (Chopra & Thomas, 2014). Oedema is the major presenting feature of NS. It becomes evident when the fluid retention exceeds 3% of the body weight. Abdominal pain is a frequent symptom and is suggestive of hypovolaemia leading to visceral vasoconstriction.
Consequences of nephrotic syndrome
- Odema
- Cardiovascular diseases
- Risk of rickets
Management Nephrotic syndrome
Management of the nephrotic syndrome is 2-fold: treatment of symptoms and complications (ie, edema, hyperlipidemia) and treatment of the underlying disease process (eg, corticosteroid therapy for primary diseases), (Chopra & Thomas, 2014).
Therapies for the nephrotic syndrome
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARBs) for reducing proteinuria and controlling blood pressure.
- A low-sodium (<2 g/24 hours) diet and diuretic therapy for control of edema.
- The use of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors (ie, statins) may be effective in treating the dyslipidemia.
- Protein requirements should be 0.8-1.0 g/Kg body weight
- Low fat intake, especially for fat that have no trans-fats
- Restriction of diets high in sodium and potassium
- Use of vitamin D, B6, B12, folate, copper, zinc, iron and calcium supplements
Chronic kidney disease (CKD)
CKD is defined as a reduced glomerular filtration rate, increased urinary albumin excretion, or both, (Jha et al., 2013). It is a condition of decreased kidney function shown by glomerular filtration rate (GFR) of less than 60 mL/min per 1·73 m², or markers of kidney damage, or both, of at least 3 months duration, (Jha et al., 2013) (Webster, Nagler, Morton, & Masson, 2016). Prevalence is estimated to be 8–16% worldwide.
Chronic kidney disease stages
According to Chawla, Eggers, Star, Kimmel, & Ingelfinger, (2014) the development of CKD progresses through the following stages:
Table 2. Stages of CKD
| Stage | Definition | GFR (ml/min/1.73 m2) |
| 1 2 3A 3B 4 5 | Kidney damage with normal GFR Kidney damage with mild decrease in GFR Mildtomoderate decrease in GFR Moderatetosevere decrease in GFR Severe decrease in GFR Endstage renal disease | ≥90 60–89 45–59 30–44 15–29 <15 |
Causes of chronic kidney disease
Diabetes mellitus (DM) is one of the leading causes of chronic kidney disease (CKD) which eventually leads to insulin resistance and decreased insulin degradation (Rajput, Sinha, Majumdar, Shunmugavelu, & Bajaj, 2017). According to Beto, Fand, Pharmd, & Bansal, (2014), CKD may be caused by:
- Diabetes,
- Hypertension, or
- Glomerulonephritis
Other problems that can cause kidney failure include:
- Autoimmune diseases, such as lupus and IgA nephropathy
- Genetic diseases (diseases you are born with), such as polycystic kidney disease
- Nephrotic syndrome
- Urinary tract problems
Chronic kidney disease Symptoms
- Most patients are asymptomatic.
- Some may present with symptoms of fatigue due to underlying anemia.
- Others may present with nonspecific nausea, vomiting, and decreased appetite due to uremia.
- Urine output typically does not change until kidney failure (stage 5/G5).
Diagnosis for chronic kidney disease
Diagnoses is done using a physical examination and tests to check kidney function. These include:
- Urinalysis
- Serum creatinine test
- Blood urea nitrogen test
- Estimated glomerular filtration rate (GFR)
Telemedicine has significant potential to extend nephrology consultation to rural and isolated communities (Narva, Romancito, Faber, Steele, & Kempner, 2017).

Slowing Progression of chronic kidney disease
The diet is still at the center of management of many kidney diseases. To effectively slow down progression of CKD to ESRF:
- Control of diabetes should be aggressive in early CKD;
- Blood pressure control (Sign, 2013).
- Several studies suggest a possible benefit of oral bicarbonate therapy in slowing CKD progression
- Obese patients should lose weight.
- Management of traditional cardiovascular risk factors
Dietary Management for chronic kidney disease
The following dietary management practices (Beto et al., 2014; Hynes, Nicolas, & Lew, 2017) are deemed ideal:
- If the patient has stage 3 CKD and has normal potassium levels without a history of hyperkalemia, consider encouraging the patient to eat more fruits and vegetables. The goal should be 5 servings/day, total, of fruits and vegetables.
- Advise against excessive protein intake. 0.6 to 0.8 g/kg of BW/day with at least 50% high biological value protein to potentially slow disease progression (particularly in patients with diabetes) and achieve/ maintain adequate serum albumin.
- Patients to limit sweets and refined grains, such as white bread, cakes, and cookies, opting for whole grains instead. Target 25 to 35 kcal/kg of BW/day to achieve or maintain goal body weight.
- Advise caution with foods and drinks high in phosphorus, such as dark sodas and processed foods. At ESRF, levels of 800 to 1,000 mg/day to achieve goal serum level of 3.5 to 5.5 mg/dL or below; coordinate with oral phosphate binder prescription.
- Encourage healthier fats. Saturated fats should be same as for general population; <7% of total fat.
- Barring no fluid restrictions, patients to drink 1–2 l of water per day. Patients should also avoid fruit juices, dark sodas, and added cream, milk and sugar in tea/coffee.
- Encourage 5–10% weight loss in obese patients with stages 1–3 chronic renal failure. Exercises that increase muscle mass, such as squats, are helpful.
- Moderate alcohol intake (5 glasses of wine per week) is acceptable
- Always refer patients to tobacco cessation programs when needed Key points
- Salt should be limited to no more than 2.4 g/day
Treatment for chronic kidney disease
Treatment may include:
- Dialysis
- Hemodialysis, which uses a machine to process your blood.
- peritoneal dialysis, involves using the lining of the abdomen to filter blood inside the body using a catheter.
- Kidney transplant: removing your affected kidneys (if removal is needed) and placing a functioning donated organ.
- Drugs: angiotensin converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs).
Renal replacement therapy in management of kidney diseases
Renal replacement therapy is therapy that replaces the normal blood-filtering function of the kidneys. There are several different modalities of RRT, and each has potential advantages and dis- advantages depending on the clinical situation. In hemodynamically unstable patients, continuous RRT (CRRT) has become the standard of care. The established target dose for CRRT is a delivered effluent rate of 20 to 25 mL/kg/h. To achieve this, a prescribed dose of 25 to 30 mL/kg/h may be required (Heung & Yessayan, 2017).
Dialysis in management of kidney diseases
Dialysis initiation should be considered when GFR is 10 mL/min/1 .73 m2• Studies suggest that the wellselected patient without overt uremic symptoms may wait to initiate dialysis until GFR is closer to 7 mL/min/1 . 73 m2. Other indications for dialysis, which may occur when GFR is 10-15 mL/min/1 .73 m2 include uremic symptoms, fluid overload unresponsive to diuresis, and refractory hyperkalemia.
Two types of dialysis may be performed depending on the patient:
- Hemodialysis
- peritoneal
Kidney transplantation in management of kidney diseases
Renal transplantation is well established as the treatment of choice for selected patients with end-stage renal failure. Occasionally, transplantation may also be considered in patients with an eGFR >15 ml/min/1.73 m2 if they are suffering significant uraemic side effects (Barlow, 2017). Multiple studies have demonstrated that patient survival is better with renal transplantation than on dialysis. A renal transplant recipient can therefore enjoy an improved quality of life whilst benefiting from a reduction in the mortality compared with long-term dialysis. However, the success of transplantation is limited by the disparity between an ever-growing demand and an insufficient supply of organs (Barlow, 2017).
Sources of kidneys for transplantation
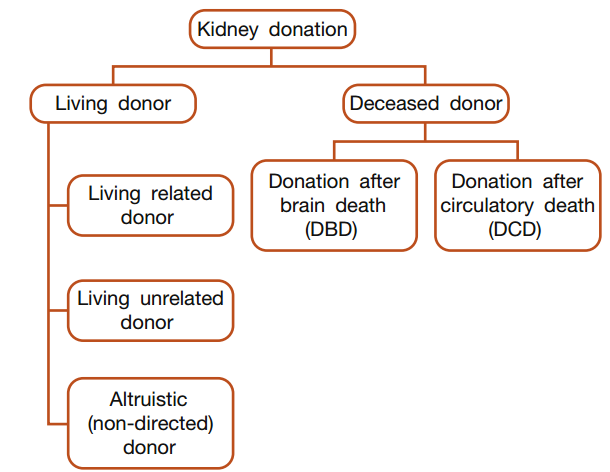
Conclusion
Many kidney diseases result in reduced or loss of kidney function – affected glomeruli. Prevention is possible with administration of specific diet choices – Lifestyle choices. Treatment may range from drug use to minor surgeries to renal replacement therapies.
It is essential for individuals with kidney diseases to work closely with healthcare professionals, including nephrologists and registered dietitians, to develop an individualized diet plan that meets their specific needs. This plan should consider the stage of kidney disease, nutritional requirements, and other underlying health conditions. Regular monitoring of kidney function and adjustments to the diet may be necessary as the condition progresses.
Below is a slide show of the above article. Click on the next button or inside the slide to see the next slide!
References
- Barlow, A. D. (2017). Kidney transplantation. Surgery, 35(7), 378–384. https://doi.org/10.1016/j.mpsur.2017.04.002
- Beto, J. A., Fand, R. D. N., Pharmd, W. E. R., & Bansal, V. K. (2014). Medical Nutrition Therapy in Adults with Chronic Kidney Disease: Integrating Evidence and Consensus into Practice for the Generalist Registered Dietitian Nutritionist. Journal of the Academy of Nutrition and Dietetics. https://doi.org/10.1016/j.jand.2013.12.009
- Chawla, L. S., Eggers, P. W., Star, R. A., Kimmel, P. L., & Ingelfinger, J. R. (2014). Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. The New England Journal of Medicine, 371, 58–66. https://doi.org/10.1056/NEJMra1214243
- Chen, H., & Busse, L. W. (2017). Novel Therapies for Acute Kidney Injury. Kidney International Reports, 2(5), 785–799. https://doi.org/10.1016/j.ekir.2017.06.020
- Chopra, B., & Thomas, L. (2014). Nephrotic Syndrome. Hospital Medicine Clinics, 3(2), e245–e254. https://doi.org/10.1016/j.ehmc.2013.11.008
- Han, H., Segal, A. M., Seifter, J. L., & Dwyer, J. T. (2015). Nutritional Management of Kidney Stones ( Nephrolithiasis ). Clinical Nutrition Research, 4, 137–152.
- Heung, M., & Yessayan, L. (2017). Renal Replacement Therapy in A cute Kidney I n j u r y : Controversies and Consensus. Critical Care Clinics, 33(2), 365–378. https://doi.org/10.1016/j.ccc.2016.12.003
- Hynes, M., Nicolas, N., & Lew, S. (2017). Emerging concepts: the dietary treatment of chronic kidney disease. Journal of Kidney Care, 2(3).
- Janssen, M. J., Arcolino, F. O., Kok, R. J., & Mastrobattista, E. (2016). Author ’ s Accepted Manuscript Gene based therapies for kidney regeneration. European Journal of Pharmacology. https://doi.org/10.1016/j.ejphar.2016.07.037
- Jha, V., Garcia-garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., … Yang, C. (2013). Global Kidney Disease 3 Chronic kidney disease : global dimension and perspectives. The Lancet, 6736(13), 1–13. https://doi.org/10.1016/S0140-6736(13)60687-X
- Liebeskind, D. S. (2014). Nephrotic syndrome. Neurologic Aspects of Systemic Disease Part I (1st ed., Vol. 119). Elsevier B.V. https://doi.org/10.1016/B978-0-7020-4086-3.00026-6
- Meersch, M., Volmering, S., & Zarbock, A. (2017). Prevention of Acute Kidney Injury. Best Practice & Research Clinical Anaesthesiology. https://doi.org/10.1016/j.bpa.2017.08.002
- Narva, A. S., Romancito, G., Faber, T., Steele, M. E., & Kempner, K. M. (2017). Managing CKD by Telemedicine : The Zuni Telenephrology Clinic. Advances in Chronic Kidney Disease, 24(1), 6–11. https://doi.org/10.1053/j.ackd.2016.11.019
- Rajput, R., Sinha, B., Majumdar, S., Shunmugavelu, M., & Bajaj, S. (2017). Consensus statement on Insulin Therapy in Chronic Kidney Disease. Diabetes Research and Clinical Practice. https://doi.org/10.1016/j.diabres.2017.02.032
- Shetty, M. S., Yang, L., Bagga, A., & Chakravarthi, R. (2017). Prevention and Therapy of AKI in the Developing World. Kidney International Reports. https://doi.org/10.1016/j.ekir.2017.03.015
- Silver, S. A., & Siew, E. D. (2017). Follow-up Care in Acute Kidney Injury : Lost in Transition. Advances in Chronic Kidney Disease, 24(4), 246–252. https://doi.org/10.1053/j.ackd.2017.05.008
- Webster, A. C., Nagler, E. V, Morton, R. L., & Masson, P. (2016). Chronic Kidney Disease. The Lancet, 6736(16), 1–15. https://doi.org/10.1016/S0140-6736(16)32064-5
- Zolotas, E., & Krishnan, R. G. (2016). Nephrotic syndrome. Paediatrics and Child Health, 4–7. https://doi.org/10.1016/j.paed.2016.04.006
- Figure 1 and 2. Sorced from: OpebStax CNX. Located at: http://cnx.org/contents/GFy_h8cu@9.87:PixBa–0@8/The-Kidneys-and-Osmoregulatory
- Figure 3. RMA, (2017). Renal Medicine Associates, Located at: http://renalmed.com/kidney-stones/
Our Blog ↗
Read the latest from our blog
Ask a Question ↗
Ask a question and get answers from our community
Give Feedback ↗
We value your feedback.