Introduction
There are two main types of biopolymers produced by microorganisms that survive extreme conditions. These are the extracellular polysaccharides or exopolysaccharides (EPSs), considered as a protection against desiccation and predation, and the endocellular polyhydroxyalkanoates (PHAs) that provide an internal reserve of carbon and energy [1].
Exopolysaccharides (EPSs) are high molecular weight and biodegradable polymers that are biosynthesized by a wide range of microorganisms [2], [3]. The capacity of strains to produce EPS is widespread among species of lactic acid bacteria and bifidobacteria, [4] marine bacteria like Bacillus, Halomonas, Planococcus, Enterobacter, Alteromonas, Pseudoalteromonas, Vibrio, Rhodococcus, Zoogloea as well as Archaea such as Haloferax and Thermococcus [5] and rhizobia species [3].
Food safety knowledge is for all!

Every consumer deserves to have high quality and safe food. …Read more!

EPSs are secreted by microorganisms for their survival in harsh environmental conditions as a protective mechanism [1], [6] rather than as energy sources [2]. Extreme environments, generally characterized by atypical temperatures, pH, pressure, salinity, toxicity, and radiation levels, are inhabited by these extremophiles [1]. EPSs are produced in response to biotic stress (e.g., competition), abiotic stress factors (e.g., changes in temperature, light intensity, pH, salinity) as a strategy of adaptation, like in the case of acidophilic or thermophilic species belonging to the bacteria and archaea species [7], [8]. For instance, cyanobacteria are major primary producers in extreme cold ecosystems [9] while in microalgae, EPS production was observed during all growth phases but increased during the stationary phase [10].
EPSs can be classified into two groups. These are homopolysaccharides and heteropolysaccharides. Homopolysaccharides are polymers which are composed of one type of monosaccharide, e.g., dextran. Heteropolysaccharides are polymers of repeating units that are composed of two or more types of monosaccharides, e.g. D-glucose, D-galactose, D-mannose, etc. Other organic or inorganic substituents, such as phosphate, sulfate, succinic, acetic and pyruvic acids can also be found in EPSs [2], [5], [6].
Microbial EPSs generally exist in two forms depending on their locations: cell-bound EPSs which closely adhere to the bacterial surface, as capsular, and released EPSs that are released into the surrounding medium, as free EPSs. EPSs produced from LAB are distinguished as ropy or non-ropy EPS [2]. EPSs are important components of the matrix formed (biofilms) by bacteria for protection among other functions [11].
Functions of exopolysaccharides in bacterial cells
| Process | Functional relevance |
| Adhesion | Initial step in colonization to biotic and abiotic surface |
| Aggregation of bacterial cells and formation of biofilms | Intermediation in upkeep of the mechanical stability of biofilms |
| Immobilization of mixed bacterial populations Generation of a meium for commnication processes | |
| Protective barrier | Resistance to nonspecific and specific host defenses (immune response, protection against free radical generation) |
| Resistance to certain biocides and antibiotics | |
| Resistance to bacteriophages (a bacterial surface receptor is masked by EPS) | |
| Sorption of exogenous organic compounds | Scavenging and accumulation of nutrients from the environment |
| Sorption of xenobiotics (detoxification) | |
| Sorption of inorganic ions | Accumulation of toxic metal ions (detoxification) |
| Promotion of polysaccharide gel formation | |
| Retention of water | Prevention of desiccation under water-deficient conditions |
| Nutrient source | EPS as source of carbon, nitrogen and phosphorus |
| Interaction with enzymes | Accumulation/retentions and stabilization of secreted enzymes |
Types of exopolysaccharides and their Composition
Polysaccharides are present in all organisms and exhibit a great variety of biochemical structures based on glycosidically-linked combinations of up to 40–50 different monosaccharides (hexoses and pentoses) including many complex sugars [10]. Examples of various types of EPSs is given below:
- Dextran – Leuconostoc mesenteroides and Leuconostoc mesenteroides
- Mutan – Streptococcus mutans and Streptococcus sobrinus
- Alternan – Leuconostoc mesenteroides
- Reuteran – Lactobacillus reuteri
- Others include
- Dextran
- Alternan
- Reuteran
- Levan
- Inulin
- Kefiran
- Oligosaccharides
- Glucan
- β and α glucans
Exopolysaccharides biosynthesis
The biosynthesis of EPSs occurs in different growth phases, and depends on environmental conditions and the organism used for the production [3]. For instance, Lactobacillus sp. were found to synthese EPS mainly during the exponential growth phase [12]. The homopolysaccharides are synthesized by extracellular enzymes which are secreted from the bacteria or located on the cell surface whereas the synthesis of heteropolysaccharides is complex.
The synthesis of EPSs involves a larger number of genes coding for enzymes and regulatory proteins. The assembly of basic repeating unit occurs at the cytoplasmic membrane. Once the basic repeating unit is assembled, polymerization may take place outside the cell. Then, the EPS may be covalently linked to the cell surface to form a capsule, or released into the medium as slime.
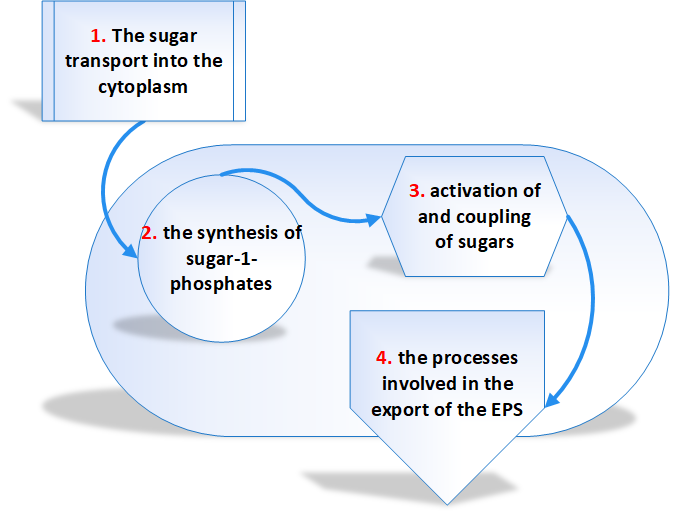
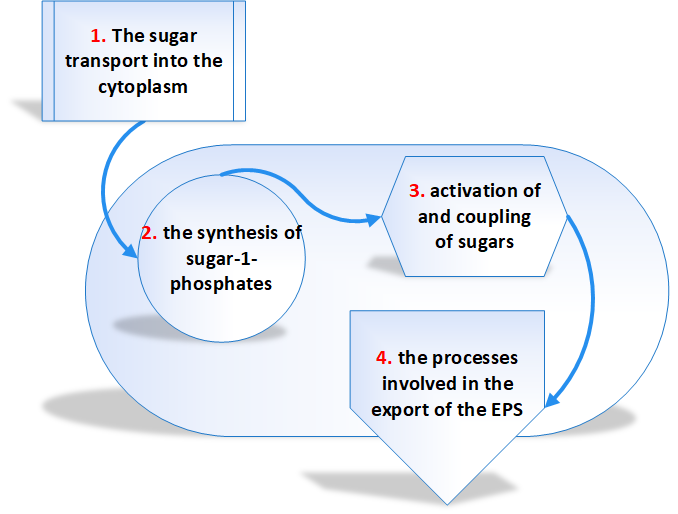
The biosynthetic pathway is divided into four separate reaction sequences [3]. These are
- The sugar transport into the cytoplasm – The monosaccharides and disaccharides are the principal carbon sources of microorganisms.
- The synthesis of sugar-1- phosphates – The majority of the sugar molecules entered into the cytoplasm of bacteria are phosphorylated into sugar-6-phosphates and degraded through glycolysis.
- Activation of and coupling of sugars – The genes involved in EPS biosynthesis are classified into 2 groups. Those genes required for the synthesis of sugar nucleotides belong to first group and the second group genes are those which are specific to EPS. The majority of EPSs are synthesized from glucose, galactose, and rhamnose precursors all synthesized from glucose-1-phosphate.
- The processes involved in the export of the EPS – It has been assumed that the polymerization and export of EPS requires the action of a flippase to translocate the lipid-bound repeat units, a polymerase to catalyze the coupling of repeat units and finally an enzyme to catalyze the detachment of the lipid-bound polymer.
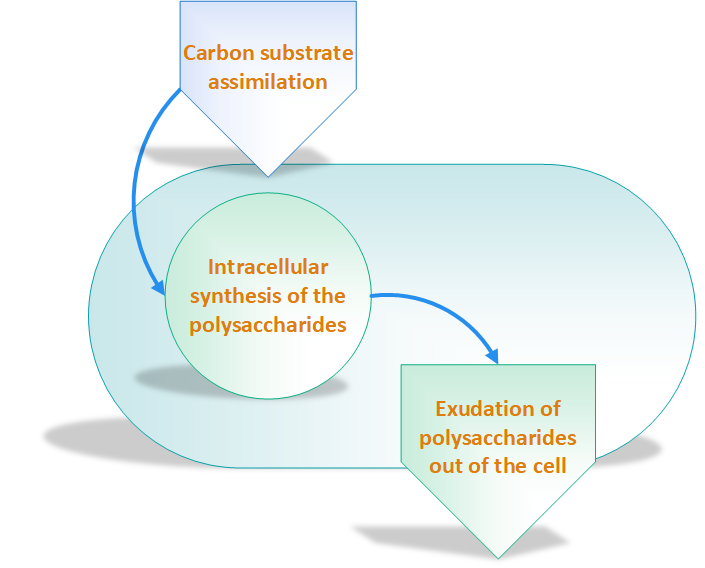
According to Sanlibaba & Çakmak, [2], incubation temperature and time, growth medium, acidity of growth medium and type of strain have an impact on EPSs production. Other researchers have categorized EPSs biosynthesis three main steps as shown in figure 2 [7]:
Exopolysaccharides Production
Although the ability to secrete exopolysaccharides (EPS) is widespread among microorganisms, only a few bacterial (xanthan, levan, dextran) and fungal (pullulan) EPS have reached full commercialization [8].
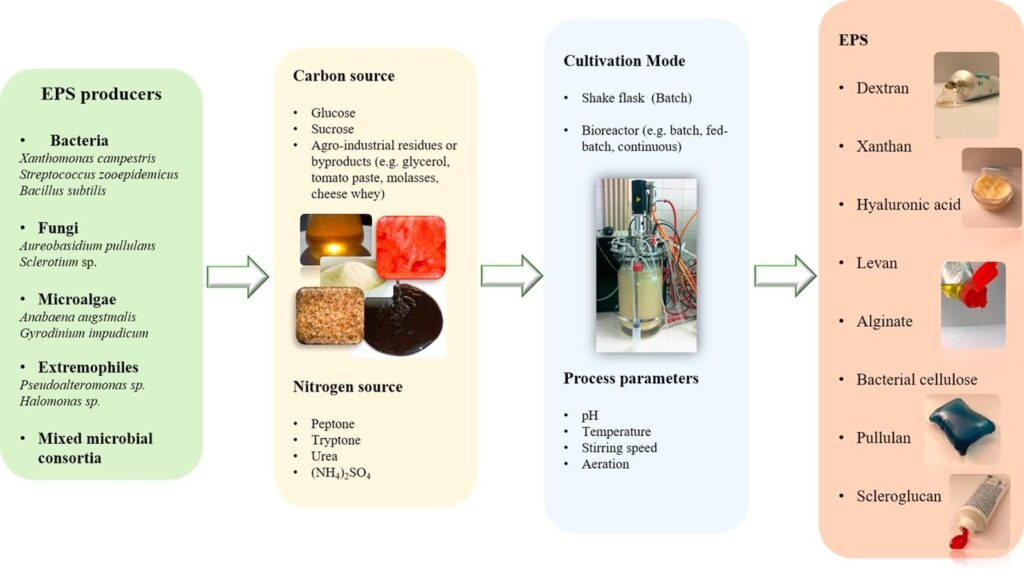
Producers of exopolysaccharides
Bacteria
The ability to secrete polysaccharides is broadly distributed among bacteria, including species of the Genera Streptococcus, Xanthomonas and Acetobacter. E.g. Dextran, produced by lactic acid bacteria of the genera Leuconostoc, Streptococcus, Weisella, Pediococcus and Lactobacillus. Dextran, gellan gum and curdlan have been approved by the FDA (Food and Drug Administration), for use in food [8].
1.5.1.2 Fungi and yeast
EPS production is widely distributed among fungi, including members of the Genera Aureobasidium, Candida, Cryptococcus. E.g. pullulan [8].
Microalgae
Microalgae species mainly researched on are Arthrospira platensis, Aphanizomenon, Chlorella vulgaris, Dunaliella salina and Porphyridium cruentum [8].
Mixed microbial consortia
Several processes involving various bacteria occurring in the same system have also been researched on for the production of EPS [8]
Production Conditions
There is no general cultivation conditions ideal for all microbial producers. Different microorganism will yield different quantities at different production conditions. For instance, production by bacteria may range between 0.29 and 100 g/L, in processes taking 0.5 – 7 days while fungi take longer times (2 – 32 days) with possibility of lower volumetric productivities [8]. Carbon availability concomitant with limiting nitrogen is usually reported as favoring EPS production by microorganisms. Under growth limiting conditions, the carbon source is derived towards polysaccharide synthesis [8], [10] However there are exceptions, with increasing nitrogen. Glucose and sucrose are the most commonly used carbon sources for microbial cultivation and production of EPS for most microorganisms. Other elements, such as phosphorus, potassium and metal cations, are also required for microbial growth and EPS synthesis. Clean nutrient sources can be used but some researchers also report the use of waste in production of EPS [8]. Other cultivation parameters of interest include:
- pH,
- temperature,
- Dissolved Oxygen concentration
- stirrer speed
- Mixing and aeration
Cultivation Methods
At lab scale, batch shake flask cultures are usually used initially to define nutritional requirements and culture conditions. Small bioreactors with similar design as those of the large scale production fermenters should be used [8]. Batch cultures strategies, with a growth phase in nutrient replete condition, followed by a production phase under deprivation condition can be used [10]. Other cultivation modes include fed-batch and continuous culture [8].
Bioreactor design
Stirred tank reactors (STRs) are the most utilized fermenters at both lab and industrial scale. The two most commonly used fermenter configurations for microbial cultivation are the continuous STR (CSTR) and the air-lift reactor (ALR). Other fermenter configurations have been used for EPS production by different microorganisms. For example, continuous production of levan in a packed-bed bioreactor [8].
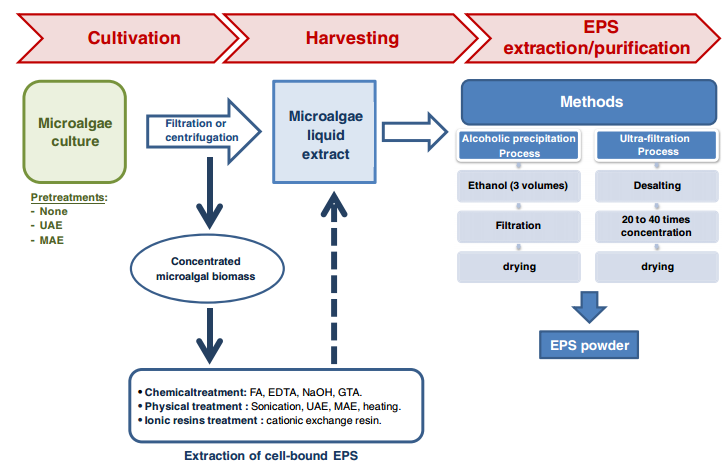
Downstream processing/ Recovery
The specific method used for recovery of EPS from the cultivation broth depends on characteristics of the producing organisms, the type of polysaccharide and the desired degree of purity. Commonly, the downstream processing involves [13], [14], [5], [8] the following processes:
- cell removal by centrifugation or filtration,
- recovery of the polymer from the cell- free supernatant through precipitation
- The precipitate is then separated from the solvent-water mixture and dried.
Several additional procedures can be used to remove contaminants,
- re- precipitation with diluted aqueous solutions,
- deproteinization by chemical or enzymatic methods and membrane processes
Examples of exopolysaccharides production:
1.5.7 Scale up
Elective methods for improving the commercial scale production and field application of microbial biopolymers [5],[2],[4] are;
- optimizing the fermentation conditions,
- biotechnological tools involving genetic and metabolic engineering,
- the exploration of cheap fermentation substrates for their production
Genetics of exopolysaccharides Production
Production of EPS is influenced both various sets of genes. There are genes that code for the production of the precursors required in the production of EPS and those that code for the specific EPS manufacture. Genetic engineering can therefore be applied to influence the types and quantities of EPS produced. Furthermore, nonproducing generally recognized as safe (GRAS) bacteria can be engineered to produce the much needed EPS [3], [6].
Uses of exopolysaccharides in Food and Nutrition
According to [6] and [2] bacterial EPSs have possible commercial applications in
- Pharmaceutical industry,
- Food processing,
- Drug detoxification,
- Bioremediation,
- Cosmetics
- Bioflocculants,
- Bio-absorbents,
- Heavy metal removal agents,
- Drug delivery agents,
The most used and patented bacterial EPSs are xanthan, cellulose, gellan, alginate, etc [6]. In the food industry [2] microbial EPSs can be used in
- control viscosity and modify flow
- Improve texture, mouth feel
- Thickeners,
- Suspending agents,
- Salad dressings,
- Frozen food icing,
- Moisturizing agents
Inaddition, they have been reported to have key impact in enhancing food nutrition:
- Production of low calories food products,
- Provision of dietary fibers
- As prebiotics which are useful in the body
- enhancing freeze-thaw stability to ensure retention of nutrients
- Films and coating agents to prevent loss of nutrients
Opportunities for further research
According to [6] downstream processing for purification and genetic engineering for increased EPS biosynthesis require more attention. Research into utilization of low cost substrates or wastes and improving downstream processing operations are also key [2].
Conclusion
EPSs are secreted by microorganisms for their survival in harsh environmental conditions especially for protection. Nevertheless, their application in the food industry in enhancing food quality as well as nutrition continues to be explored. Bacterial EPSs have possible commercial applications in many industrial processes Different microbial species can produce EPS depending on the cultivation conditions. There exists an opportunity to enhance production of EPS through biotechnological methods.
Our Blog ↗
Read the latest from our blog
Ask a Question ↗
Ask a question and get answers from our community
Give Feedback ↗
We value your feedback.


canada drugs without perscription
canada drug
canadian rx pharmacy online
best online pharmacies canada
2 For each 10 mm Hg increase in levels of SBP, the increased stroke risk in whites is 8; however, a similar 10 mm Hg increase in SBP in African Americans is associated with a 24 increase in stroke risk, an impact 3 times greater than in whites cialis on line You can buy Clomiphene Citrate Clomid from our online store in the USA
canadian drug store