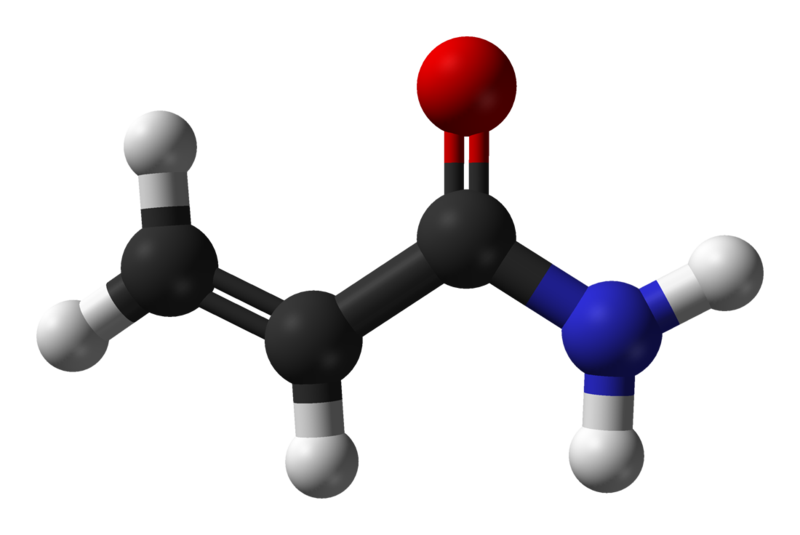
Occurrence
Normally, acrylamide is not present in raw food products; however, during thermal processing, it is produced and found in high amounts in carbohydrate-rich plant foods (potato chips, bakery items, coffee, roasted almonds, cereal products) than the protein-rich animal foods.
This colorless, non-volatile crystalline solid is produced in these foods during the heat treatments, such as baking, frying and roasting (1). Processed plant products tend to contain significant quantities due to the presence of precursors for the production of acrylamide, such as sucrose and fructose, together with amino acid asparagine (2).
Food safety knowledge is for all!

Every consumer deserves to have high quality and safe food. …Read more!

For instance, when wheat flour products are fried in vegetable oils, the acrylamide content may increase from undetectable levels to values that may exceed 350μg/kg in overheated food. An increase of about 10°C, in the oil temperature, can in fact double the content (3). Studies have shown that many commonly consumed fried and baked foods contain naturally occurring levels of acrylamide (4).
Chemistry
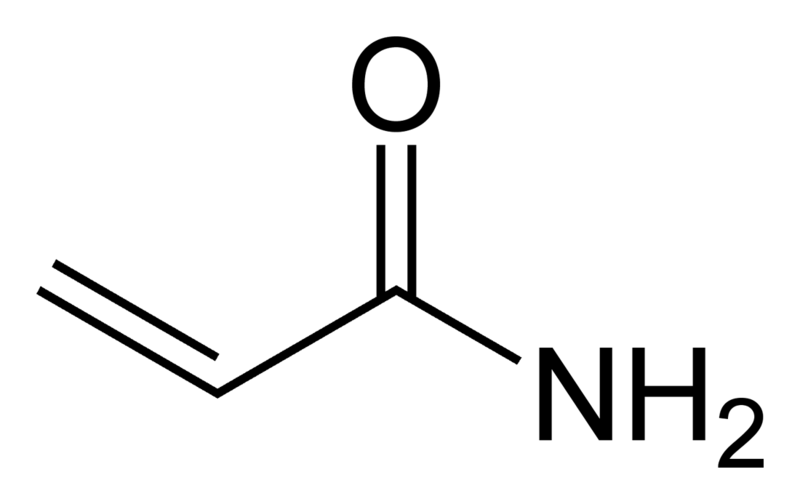
Acrylamide (C3H5NO; 2-propenamide) is a scentless, white crystalline substance with a molecular weight of 71.08. It is highly soluble in water (2155 g / l at 30 ° C) as well as polar solvents (e.g. acetone, methanol and ethanol) and not in non-polar solvents. Its density is 1.27 g / l (25 ° C), the boiling point is 136 ° C at 3.3 kPa and the melting point is between 84-85 ° C (5).
It is generated in especially starchy foods while undergoing heat treatments, such as baking, frying and roasting (1). It is formed primarily in foods by the reaction of asparagine (free amino acid) with the reducing sugars ( glucose and fructose) as part of the Maillard reaction during heating under high temperatures and low humidity, which serves as the main route for its formation (1,2,6)
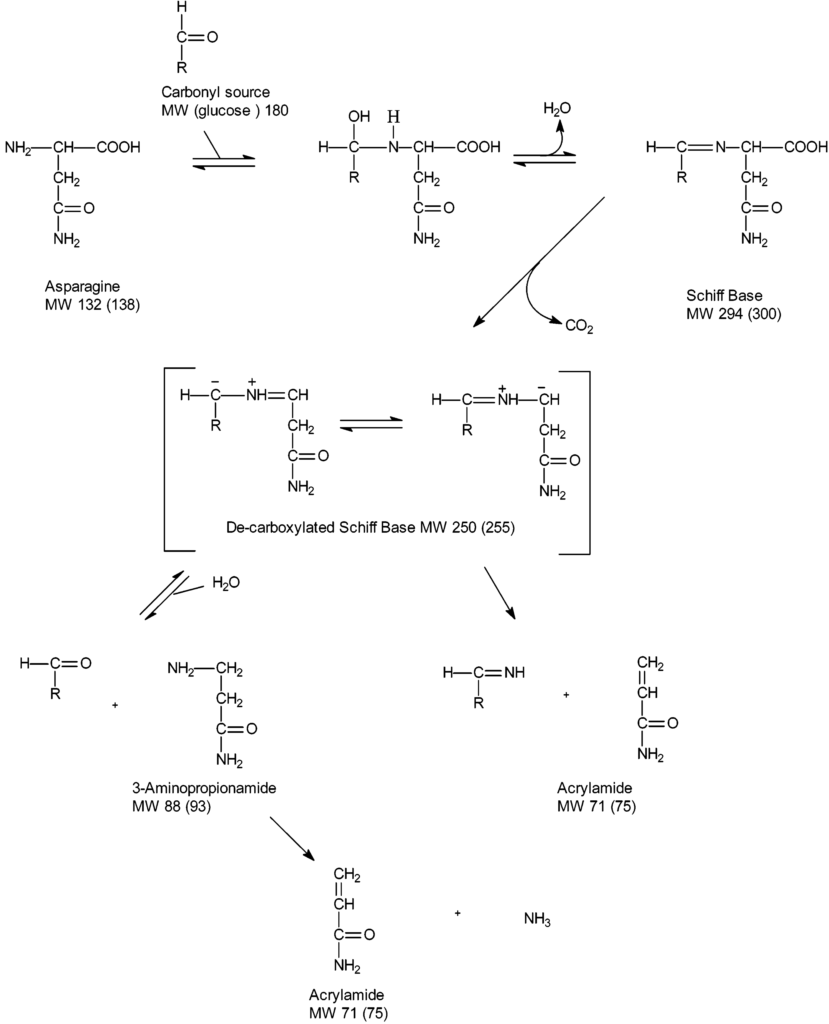
First, the amino acid asparagine may undergo condensation reaction together with reducing sugars or carbonyl compounds of the pathway followed by dehydration to schiff base compounds. It decarboxylates to unstable 5-oxazolidinone intermediates to form stable azomethineylides that undergo series of proton transfer and β-elimination to form acrylamide (2). Asparagine, is the chemical backbone of this toxin, essentially through decarboxylation and loss of nitrogen moiety.
The chemistry implicated has demonstrated the presence of various possible intermediates (decarboxylated Amadori product, 3-aminopropionamide) in the formation of acrylamide (6). Acrylamide formation increases towards the end of the frying process (1).
Mechanisms of Formation of acrylamide in food
Acrylamide formation follows different routes but, the asparagine route is the most important:
Formation via Asparagine Route
The major pathway leading to the formation of acrylamide in food is part of the Maillard reaction with free amino acids (asparagine) and the reducing sugars (glucose and fructose). Maillard reaction is a non-enzymatic browning reaction that occurs in food during baking as well as frying.
During this process, the reactive carbonyl from the maillard reaction reacts with asparagine to form a Schiff base that undergoes a variety of rearrangements as well as degradation processes to ultimately produce acrylamide. The potential for formation of acrylamide is therefore strongly related to the food product’s glucose and fructose content.
The free concentration of asparagine is the main determinant of acrylamide formation. Research has shown that the reducing sugars are the major limiting factors in tubers like potatoes, while asparagine is the major limiting factor in cereal product such as cereal bran.
Toxicology of acrylamide
Acrylamide is considered likely or potentially carcinogenic to humans, or may be metabolized by humans to potentially carcinogenic compounds. It is considered to be the most important heat-induced contaminant in bread and bakery products (1,2,8).
Dietary acrylamide intake may increase the risk of kidney and breast cancer. The daily intake in human diets was estimated to be between 0.3 and 0.8 μg per kg body weight. Under normal conditions , the average daily intake of acrylamide is approximately 0.85 μg per kg body weight (1). Management of intake levels is crucial as the overall macro and micro nutrient composition of the diet is mainly derived from food-containing acrylamide (1).
Acute Toxicity
Acute toxicity studies, the oral LD50 for acrylamide is approximately 100-150 mg/kg in mice, rabbits, guinea pigs and rats (5). Permissible levels in drinking water as established by the World Health Organization are 1 µg/ L, the U.S. Environmental Protection Agency (EPA) at 0.5 µg/L, and the European Union at 1 µg/L. Levels (4).
Neurotoxicity
The neurotoxic properties of acrylamide have been studied most because these are the only toxic effects that have been shown both in humans from occupational exposure and from studies in laboratory animals. It was found to affect transmission of nerve impulses by affecting the nerves (4).
1.3.3 Reproductive toxicity
Reproductive toxicity was also observed in laboratory animals exposed to high levels of acrylamide. The NOAEL for reproductive toxicity was estimated to be 2–5 mg/kg body weight/day depending on the endpoint of fertility or embryonic death. However, no reproductive toxicities have been reported in humans.
Genotoxicity
The genotoxicity of acrylamide and that of its major metabolite glycine has been studied. The most significant damage is considered to be a direct action on the DNA molecule causing point mutation.
Carcinogenicity
It is classified as a “probable human carcinogen”. Animals exposed to high concentrations in the drinking water for prolonged periods develop multiple tumors at multiple sites in both genders. In addition, acrylamide was shown to be genotoxic in cell culture by in vitro tests and in vivo animal models (4).
Mitigation of acrylamide in food
According to (2), mitigation in different food matrices involves:
- Selection of raw materials: Selection of potatoes and wheat based on their free asparagine and reducing sugar content, along with pre- and postharvest conditioning to reduce acrylamide formation during processing
- Blanching: Blanching of potatoes before processing leads to softening of tissues and removal of free asparagine based on solutes used during the process.
- Use of asparaginase (EC 3.5.1.1) which converts free asparagine into aspartic acid and ammonia, thereby limiting the formation of this toxin.
- Addition of additives
- Substitution of leavening and raising agents containing ammonia with sodium and potassium helps in the reduction of acrylamide in baked food products.
- Replacing reducing sugars with sucrose greatly reduces acrylamide formation.
- Addition of acidity regulators such as citric acid resulted in the reduction of acrylamide mainly due to unfavorable pH for acrylamide formation.
- Fermentation – Saccharomyces cerevisiae (baker’s yeast) removes up to 70% of acrylamide during a 48 h fermentation at 30°C in instant coffee. Filamentous fungus (Aspergillus oryzae) is used for the degradation of this toxin in food and beverage industries.
- Vacuum treatment – Dry food products such as biscuits and potato chips subjected to the following conditions (6.67 Pa, 60°C, 5–15 min), results in reduction in the acrylamide content.
Our Blog ↗
Read the latest from our blog
Ask a Question ↗
Ask a question and get answers from our community
Give Feedback ↗
We value your feedback.